Abstract
Background: Systemic mastocytosis (SM) can occur on its own or in association with non-mast cell disorders (SM with associated hematological neoplasm, SM-AHN). Because extensive bone marrow involvement by non-mast cell disease may obscure mast cells in routinely-stained biopsies, it is possible that SM-AHN is under-recognized. Given the high association between KIT D816V mutations and SM, we asked if systematic evaluation for somatic KIT mutations in patients with known or suspected hematologic malignancies could identify unsuspected cases of SM-AHN.
Methods: We queried 5754 clinical next generation sequencing (NGS) samples from 4173 unique patients with known/suspected hematologic neoplasms evaluated at our institution between 1/1/15 and 6/30/17 using a custom, 95-gene, amplicon-based NGS panel (PMID 27339098). Exons 8-9, 11 and 17 of KIT were analyzed. In patients with a somatic KIT mutation but without known SM, marrows were assessed for SM with mast cell tryptase and CD25 immunohistochemistry (IHC).
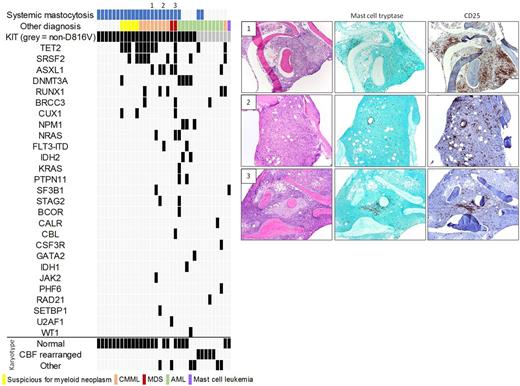
Results: We identified 37 somatic KIT mutations in 35 patients (Figure), including non-D816V (n=9) and D816V (n=26; 1 case with concurrent D816Y).
Non-D816V KIT mutations occurred in 9 patients: 5 core-binding factor-rearranged acute myeloid leukemia (CBF-AML) [418_419insFF (n=1); 417_419TYDdelinsI (n=1); Y823C (n=1); D816Y (n=2; 1 with also V559D)], 1 AML with myelodysplasia-related changes (AML-MRC; 419delD), 1 acute promyelocytic leukemia (N822K), 1 chronic myelomonocytic leukemia (R634W), and 1 mast cell leukemia (D816Y). In addition to the patient with mast cell leukemia, 2 patients with CBF-AML had known SM [418_419insFF; D816Y and concurrent V559D]. IHC performed for this study on the remaining 6 cases did not reveal multifocal, dense aggregates of abnormal mast cells.
Of the 26 cases with the D816V variant, 18 had an established diagnosis of SM [SM without AHN (n=6); SM with suspected AHN, based on marrow morphology and/or abnormal peripheral counts (n=5); SM-AHN where AHN = chronic myelomonocytic leukemia (n=6) or myelodysplastic syndrome (n=1)]. In the 3 remaining cases of CMML or MDS with KIT D816V (marked on Figure), SM was not clinically or pathologically suspected. IHC performed following identification of the KIT D816V mutation by NGS revealed clusters of spindled, CD25+ mast cells, consistent with SM-AHN. All 3 patients also had a serum tryptase >20 ng/mL and splenomegaly, although these parameters may be influenced by the AHN. In 1 patient, retrospective review of prior liver/lymph node biopsies revealed previously unrecognized aggregates of spindled, CD25+ mast cells accounting for unexplained fibrosis. The patient was started on Midostaurin following SM diagnosis with significant clinical response. The final 5 cases with the D816V variant were AML. This included 4 de novo or therapy-related AMLs in which the KIT mutation was not present at initial diagnosis. In 2 cases, the mutation appeared in peripheral blood at time of relapse after stem cell transplant (concurrent bone marrow biopsy not performed; pre-transplant marrows showed no evidence of SM by IHC). In the other 2 cases, there was no IHC evidence of SM in leukemic marrows bearing the KIT D816V mutation. The final AML was AML-MRC with known KIT mutation; increased, CD25+ mast cells were present at time of AML but were not spindled or clustered.
None of the cases of isolated SM had additional driver mutations, whereas 15 of 16 cases of known or suspected SM-AHN exhibited driver mutations in genes that are recurrently mutated in myeloid neoplasms. In the SH-AHN cases, the KIT D816V variant allele fraction varied from subclonal to equivalent to levels of other driver mutations. A group of 30 cases of CMML (n=15) and MDS (n=15) without KIT mutations were used as controls. None of these cases showed IHC evidence of SM.
Conclusion: We find that detection of the KIT D816V mutation in CMML or MDS is invariably associated with SM, even in cases in which SM is not clinically suspected. Our data strongly suggest that identification of the KIT D816V mutation in patients with a diagnosis of CMML or MDS should prompt additional testing for underlying SM given the potential prognostic and therapeutic implications.
Aster: Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees. Steensma: Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Incyte: Equity Ownership; Onconova: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Takeda: Consultancy; H3 Biosciences: Consultancy. Lindsley: Takeda Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Consultancy; MedImmune: Research Funding. DeAngelo: Celgene: Research Funding; ARIAD: Consultancy, Research Funding; Glycomimetics: Research Funding; Blueprint Medicines: Honoraria, Research Funding; Immunogen: Honoraria, Research Funding; BMS: Consultancy; Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Shire: Honoraria; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Amgen: Consultancy, Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal